Advertisement
-
Published Date
March 26, 2025This ad was originally published on this date and may contain an offer that is no longer valid. To learn more about this business and its most recent offers, click here.
Ad Text
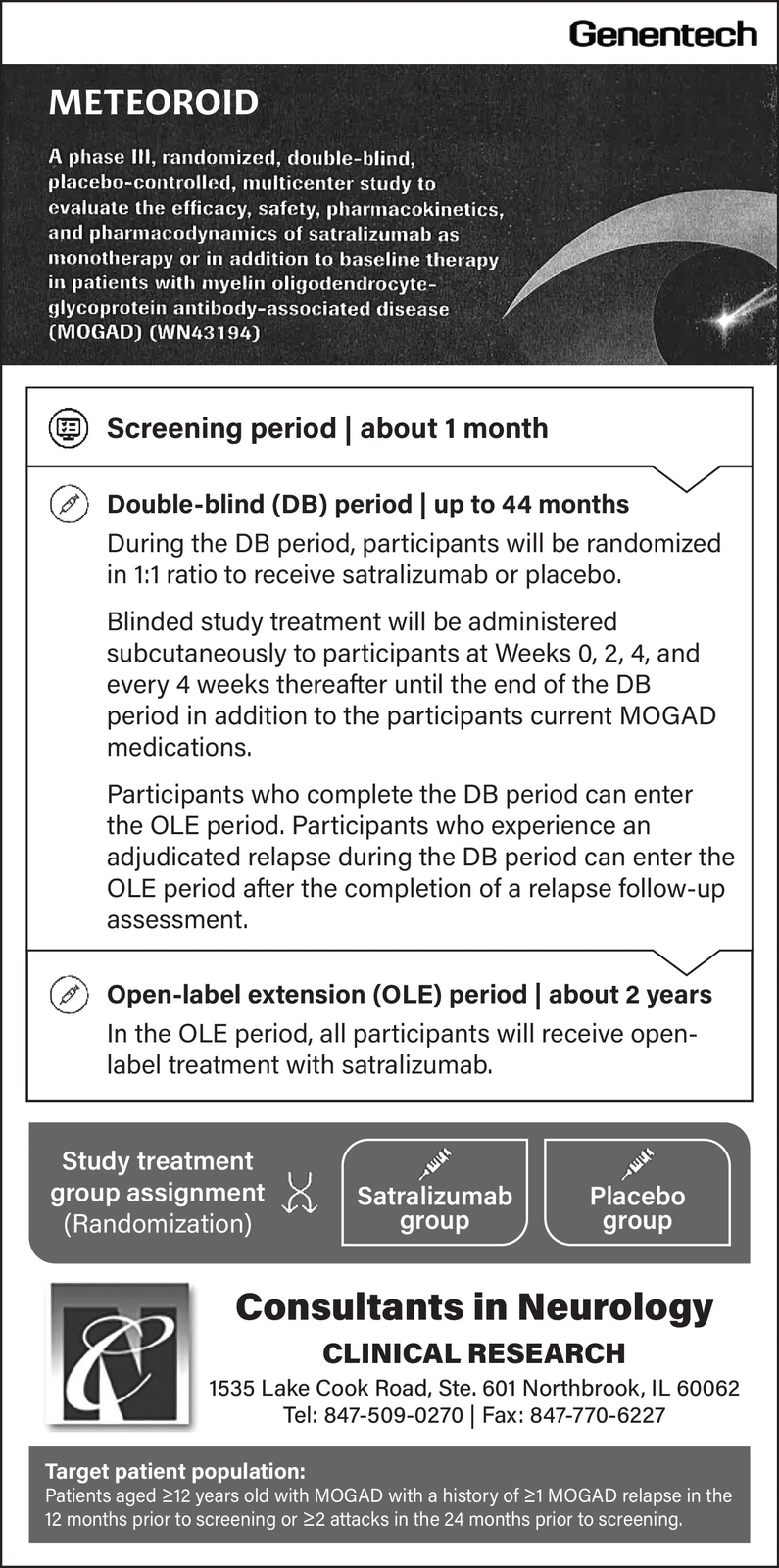
METEOROID A phase III, randomized, double-blind, placebo-controlled, multicenter study to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of satralizumab as monotherapy or in addition to baseline therapy in patients with myelin oligodendrocyte- glycoprotein antibody-associated disease (MOGAD) (WN43194) Genentech ( Screening period | about 1 month Double-blind (DB) period | up to 44 months During the DB period, participants will be randomized in 1:1 ratio to receive satralizumab or placebo. Blinded study treatment will be administered subcutaneously to participants at Weeks 0, 2, 4, and every 4 weeks thereafter until the end of the DB period in addition to the participants current MOGAD medications. Participants who complete the DB period can enter the OLE period. Participants who experience an adjudicated relapse during the DB period can enter the OLE period after the completion of a relapse follow-up assessment. Open-label extension (OLE) period | about 2 years In the OLE period, all participants will receive open- label treatment with satralizumab. Study treatment group assignment Satralizumab (Randomization) group Placebo group Consultants in Neurology CLINICAL RESEARCH 1535 Lake Cook Road, Ste. 601 Northbrook, IL 60062 Tel: 847-509-0270 | Fax: 847-770-6227 Target patient population: Patients aged 12 years old with MOGAD with a history of 1 MOGAD relapse in the 12 months prior to screening or 2 attacks in the 24 months prior to screening. METEOROID A phase III , randomized , double - blind , placebo - controlled , multicenter study to evaluate the efficacy , safety , pharmacokinetics , and pharmacodynamics of satralizumab as monotherapy or in addition to baseline therapy in patients with myelin oligodendrocyte- glycoprotein antibody - associated disease ( MOGAD ) ( WN43194 ) Genentech ( Screening period | about 1 month Double - blind ( DB ) period | up to 44 months During the DB period , participants will be randomized in 1 : 1 ratio to receive satralizumab or placebo . Blinded study treatment will be administered subcutaneously to participants at Weeks 0 , 2 , 4 , and every 4 weeks thereafter until the end of the DB period in addition to the participants current MOGAD medications . Participants who complete the DB period can enter the OLE period . Participants who experience an adjudicated relapse during the DB period can enter the OLE period after the completion of a relapse follow - up assessment . Open - label extension ( OLE ) period | about 2 years In the OLE period , all participants will receive open- label treatment with satralizumab . Study treatment group assignment Satralizumab ( Randomization ) group Placebo group Consultants in Neurology CLINICAL RESEARCH 1535 Lake Cook Road , Ste . 601 Northbrook , IL 60062 Tel : 847-509-0270 | Fax : 847-770-6227 Target patient population : Patients aged 12 years old with MOGAD with a history of 1 MOGAD relapse in the 12 months prior to screening or 2 attacks in the 24 months prior to screening .
